510K
How to Prepare a Medical Device 510k Submission for FDA | Rob Packard | Joe Hage
MedicalMarcom
9K
31,562
6 лет назад
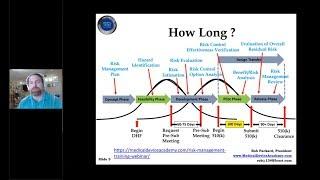
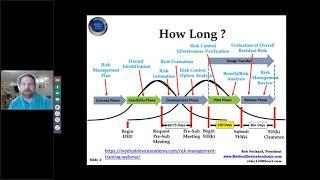
10 Steps to Preparing Your 510(k) Submission (and How to Avoid the Common Pitfalls)
Greenlight Guru
152
507
7 месяцев назад
How to Prepare a Medical Device 510k Submission for FDA | Rob Packard | Joe Hage | Updated
MedicalMarcom
848
2,825
5 лет назад
Magstim Announces FDA (510k) Clearance of rTMS Rapid2 Therapy System
Magstim Company Limited
551
1,838
9 лет назад
FDA guidance for the content of human factors in medical device 510k submissions
Medical Device Academy
235
784
2 года назад
Using the new eSTAR templates for a 510(k) submission and the FDA eSTAR draft guidance
Medical Device Academy
2K
5,123
3 года назад
What is a 510k? When Do I Need One? / Medical Device Regulations
The BME Life
109
364
8 месяцев назад
The FDA 510k Program. #fda #510k #facts #science #scientificbreakthrough #fdacleared #presubmission
Medical and Health Tips (MHT)
207
689
9 лет назад
Key differences between 510(k) and PMA pathways at the FDA
Stanford Biodesign
4K
12,509
10 лет назад
Сейчас ищут
510K
Ambiyah
Trendz
Clarkcountytoday
Techno Insights
Python Lernen Deutsch
Elder Scrolls Meme
Santosh Bishnoi Official
Valorant Funny Montage
No Boot Device Restart Your System
Kurs Lightroom Classic Cc
Asbcast
تحدي
Xxnxx 18
Waff
Anthem Info
Картинки С Днем Рождения Подруге
Waz
Maphack Dota 2
Притворился Девочкой
Rare Amazon Creatures
Car
Rimworld Alpha 17 Part 1
Rsc
Галыгин Суслик Личность
Why Is It Not Meta
Fbs
Dominic Fike Indie Guitar Type Beat
Enchantress Гайд
R Nait
Rfm Royalty Free Music Library
Pixel Gun 3D Mod Menu
510K смотреть видео. Рекомендуем посмотреть видео How to Prepare a Medical Device 510k Submission for FDA длительностью 11:06. Invideo.cc - смотри самые лучшие видео бесплатно