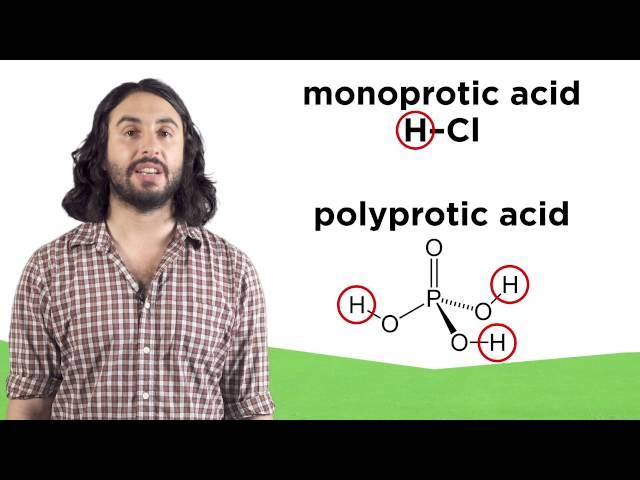
Комментарии:

❤❤
Ответить
Lich chgitein inch er. Apply sodium bicarbonate mixed with water on your hands every day. It treats a thing or two.
Ответить
you my friend help compensate for shit teachers around the world
Ответить
😅
Ответить
i love you
Ответить
why are the definitions of acid and base so H2O-centric?
Ответить
Hey Prof Dave, I'm a pre-med student planning to take the MCAT early next year. Your videos are making the review process straightforward. Thank you for what you do!
Ответить
Professor Dave,
I love you. Please teach me more chemistry.
Thank you.

Please tell the answer of the given question?
Why aniline is more basic than ammonia in gas phase but less basic than ammonia in liquid phase?

When I went to a part 141 flight school, no one told me I needed to take biology
Ответить
Thank you Prof.Dave this has helped me a lot
Ответить
I have a question
Is there a chemical that is considered a weak basic oxide?
Most of the basic oxide I researched is mostly strong.
Thank you for reply!

Sir, if oxygen has one bond and three lone pairs, as in hydroxide ion, it will have a formal charge of-1. From where does this one additional charge or one extra electron come? Also in the case of carbonate ion two of the oxygen atoms are single covalently bonded, so if oxygen has 6 valance electrons and it is sharing its one electron in bond formation then how does the lone pair upon bond formation of oxygen atom count upto three I mean since it has to share one of its six electrons in the bond, shouldn't it have 5 electrons. How after forming a single bond this one additional electron comes from here so that it provides the atom with a negative charge?
Ответить
I didnt understand anything can u plz help to explain in brief
Ответить
By the way.... A superb teacher....
But I wanna know why this man never moves his hands in videos😂 it makes ma feel weird.

Which means that HAt has stronger acidity than HI
Ответить
his little intro music makes me dance every time
Ответить
You are the most helpful outside-of-class resource for Ochem that I've found, by far! I really appreciate the amount of effort you put into all of your videos. You are the bomb!
Ответить
Sir i had one more doubt that can you explain what is protonation
Ответить
Sir can you tell why when hcl and water reacts than why hydroxide ion is not released and only hydronium and cl ion is released and not hydroxide from water
Ответить
Very great animated explanation.
Ответить
чому не державною?
Ответить
Very clearly explained. Thank you professor ❤❤
Ответить
I am lost, any more clarification on this please?
Ответить
Very good info, the only issue i have is how monotone your deliverance is and how it seems like you’re just dictating it from a piece of paper. But still very nice info
Ответить
I can't understand everything:(
Ответить
Thank You Professor Dave!
Ответить
Water is amphiprotic not amphoteric. Amphoteric is a metal oxide that can react either acid or base but amphiprotic means ability to loose or gain proton. Both not the same.
Ответить
Thanks for giving us short topic videos it's helping me much as I didn't expected and also you are better than my chemistry teacher
Ответить
honestly amazing. 10 min video, made 7 pages of notes from it!
Ответить
👍
Ответить
I had been struggling with understanding conjugate acids and bases. Within the first two minutes of your video, I understood a concept that I had been struggling with for weeks! Thanks Professor Dave!
Ответить
I wonder if a solution is acidic why is there p(oh)?
Ответить
Jesus, you can do miracles. Rah tnajih lynne
Ответить
THANKS I HAVE NAJAHET THE TEST BECAUSE OF YOU JESUS!!
Ответить
1. Thank you for not dressing up like a nerd (or not dressing up) like many other science tutorial videos out there. It's insulting to us in the science world.
2. I'm a senior chemical engineering student, and I watch your videos all the time. It's amazing how coming back to basics is essential to understanding more complex material. I'd even argue the basics are the more difficult to learn.
3. Thanks for your contribution to teaching science in a way that anyone can understand. Your summaries frankly cover the main points of entire courses I've taken (though I appreciate that studying and practicing the details is very important, too).

Thank you so much 🥰
Ответить
Thanks!
Ответить
You are amazing 😍😍
Ответить
Omg its Jesus
Ответить
Thank you chemistry jesus
Ответить