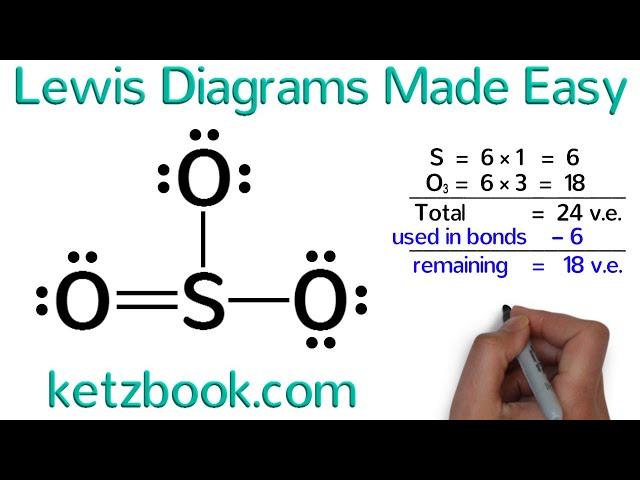
Lewis Diagrams Made Easy: How to Draw Lewis Dot Structures
Комментарии:

Dude you made it sooo easy❤️🥹🥹🥹🥹🥹, thanks 👍😍
Ответить
Thanks
Ответить
but for AlCl3 the al is unhappy, why we don't add double bond on it?
Ответить
may loe lee twy thin ny tr lr
Ответить
I just have 20 min left to go to school and i didnt do 1 chap and van take only 2 min to reach school😂 and doing this now
Ответить
that was a slay
Ответить
My chemistry teacher is useless
Ответить
Thank you sir.Just Amazing 😍
Ответить
hatss off you sir 👏you made it so easyy
Ответить
This was soooooo helpful. I was struggling with this and now I understand. THANK YOU!!!!
Ответить
Thankyou I n
Ответить
It is not a complete explanation, due to that, the SO3 structure is wrong.
Every Central Atom is allowed to break the octet rule, ie, it can have more than 8 electrons according to its maximum covalency. S can have covalencies 2, 4, 6, in this video, he made it 4, but every Central Atom wants to have a maximum colavency, which in S is 6, so it will form double bonds with all three O atoms to have a covalency of 6 and total electrons on S become 12.

Thank you Thank you Thank you
Ответить
bro you are a god
Ответить
Thanks broo❤😅
Ответить
Hydrogen share dots & become duplet not octet
Ответить
Studying one night before exam💀
Ответить
Thanks
Ответить
Me learning the night before a test be like 😭😭:
Ответить
how do i draw XeO3 using this trick?
Ответить
This was so helpful.Thank you so much☺
Ответить
Thankyou so muchhh
Ответить
Think so much it's helpful
Ответить
Bouligand
Ответить
A literal life saver💀I was taught this for a while month but could not learn it😭
Ответить
very Nicely explained
good job........
which elements have more than 8 valence electrons?
Ответить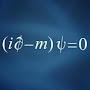
Why is Helium not in the second group (column) since it has 2 valence electrons?
Ответить
Im writing in like 20 mins bro
Ответить
Just had to subscribe...
Ответить
How do you explain the diagram of ClO3- with this method? Using all 26 electrons will complete the octet of every atom so, following this method, you shouldn't draw the double bonds; can someone explain please?
Ответить
Isn’t boron only needs 6 electrons?
Ответить
Perfect📚🖋
Ответить
Very helpful for final exam preparation. Thanks!
Ответить
Is there a periodic table that shows the Lewis Dot Structure for each element?
Ответить
That is very amazing 😍 you are very good teacher ❤please can you take about valance bound theory
Ответить
👏 👏
Ответить
last minute before my exam 😭
Ответить
Thank you so much for this video I’m studying an hour before my test and I think you just saved my grade ❤ your the best
Ответить
THANK YOU SO MUCH ❤ so clear in your explanation!
Ответить
the structure you've made for SO3 is wrong, remember that S is an exeption and can violate the octet rule which you can double bond in each Oxygen
Ответить
Really great thank you!
Ответить
Thanks a lot for the video. It was REALLY helpful!
Ответить
this dude taught me more in 7 minutes than my teacher did in a week. the public school system smh
Ответить
Thank you !
Ответить
Very interesting and helpful
Ответить
I am 2 weeks away from the AP chem test and I'm learning for the first time how to draw Lewis structures. I'm screwed.
Reply to this comment if it's been after 2 weeks and I'll let you know how I did.



![How to install VirtualBox Extension Pack [Latest] How to install VirtualBox Extension Pack [Latest]](https://invideo.cc/img/upload/MFVqM1dlY3psUlA.jpg)

![[Arknights] They nerf surtr gaming [Arknights] They nerf surtr gaming](https://invideo.cc/img/upload/SWRUOEs3bnplZ3U.jpg)