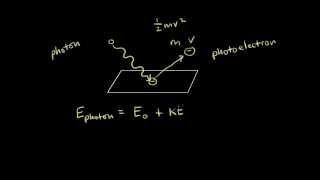
Photoelectric Effect Demonstration
Комментарии:

Basically, UV light's proton has enough energy to release electrons, while visible light cant?
Ответить
you are the best one👍👍
Ответить
Brilliant: essential, clear, no sci-fi; pure science!
Ответить
I follow your nice demonstration. I get the photoelectric effect using zinc .
But, it is not being possible using Aluminum. I think, work function of Aluminum is 4.28 eV and for Zinc this is 4.33.
What may be the problem?
Please.

I always get confused with experiments that have an entire set of assumptions in the set-up. What if no one really understands that rubbing a rod really brings electrons to the zinc? Suppose that is wrong? Is the plate touching the gold leafs or are they isolated? That's an important distinction not mentioned. Plus I think he misspoke...electrons were transferred from rod to plate.
Ответить
My physics teacher made me watch this…
Ответить
do you understend frensh?
Ответить
newdelhi retour vers marie et pierre curie
Ответить
the fact that I waited for the UV lamp to shine🤡🤡
Ответить
wow.
Ответить
Thanks very much sir am a physics students but don't understand this until I came across your video 😂😂😂 thanks Prof
Ответить
Photons are not "particles." The speed of light, as we know it, is an incomplete concept, that has never truly been measured, accounting for perception.
Perceptual light is merely an algorithmic interpretation of color spectrum frequency perturbations, as mapped by a lens.

What you say makes zero sense, and I know you're quoting Einstein who got a Nobel for this bullshit explanation. But its still a bullshit explanation. Its not even an explanation, its a contradiction in terms. A 'wave particle' is not a coherent notion and it cant be used in a coherent explanation. And I dont understand what needs explanation either. Why visible light has no effect on the electrons and invisible light has ? Because the UV light has a smaller wavelenght (10- 400nm) and can interact with the electrons, while the visible light has a longer wavelenght (400-700nm) and cant. Also higher frequency of UV light means higher probability of interaction. The electrons simply dont vibrate at the frequency of visible light, because they are invisible, so they only vibrate at the frequency of invisible light. And this is a much more coherent explanation than Einsteins. Now give my Nobel and stfu.
Ответить
What you say makes zero sense, and I know you're quoting Einstein who got a Nobel for this bullshit explanation. But its still a bullshit explanation. Its not even an explanation, its a contradiction in terms. A 'wave particle' is not a coherent notion and it cant be used in a coherent explanation. And I dont understand what needs explanation either. Why visible light has no effect on the electrons and invisible light has ? Because the UV light has a smaller wavelenght (10- 400nm) and can interact with the electrons, while the visible light has a longer wavelenght (400-700nm) and cant. Also higher frequency of UV light means higher probability of interaction. The electrons simply dont vibrate at the frequency of visible light, because they are invisible, so they only vibrate at the frequency of invisible light. And this is a much more coherent explanation than Einsteins. Now give my Nobel and stfu.
Ответить
What you say makes zero sense, and I know you're quoting Einstein who got a Nobel for this bullshit explanation. But its still a bullshit explanation. Its not even an explanation, its a contradiction in terms. A 'wave particle' is not a coherent notion and it cant be used in a coherent explanation. And I dont understand what needs explanation either. Why visible light has no effect on the electrons and invisible light has ? Because the UV light has a smaller wavelenght (10- 400nm) and can interact with the electrons, while the visible light has a longer wavelenght (400-700nm) and cant. Also higher frequency of UV light means higher probability of interaction. The electrons simply dont vibrate at the frequency of visible light, because they are invisible, so they only vibrate at the frequency of invisible light. And this is a much more coherent explanation than Einsteins. Now give my Nobel and stfu.
Ответить
Omg thank you so much for this video, it gets right to the point with a great visible demonstration!
Ответить
Thanks for this .
Ответить
What a great name for a camera assistant! Julianna Photopoulos, I hope your career in capturing light excels and brings you joy and prosperity.
Ответить
Thank you Chetan Bhagat for teaching this concept in much easier way than my teacher
Ответить
nice video!
Ответить
still not makin sense
Ответить
Hey!!...you have nailed it... 😍
Ответить
learned more in 3 min vs what my professor discussed in a week
Ответить
Wow, beautiful demonstration 😊, simply awesome💗.
I liked the way how easily entire set up is made with simple things.
My opinion:
I think Negatively charged Zinc is required so that once electron gets removed from it ,then don't come back(excess charge has tendency to go away from each other)
BUT If we started with neutral metal, then photo-electrons would have come back again to metal due to attraction of nucleus.
OVERALL enjoyed the demonstration ☺️👍👍

thank you for this video! it helps a lot.
Ответить
Wrist check ?
Ответить
Studying this for my exam tomorrow!!
Ответить
Thanks for the video. Need some explanation after reading throne of magical arcana webnovel.
Ответить
So, The higher frequency of the UV light is increasing the rate of oxidisation, right? hence the importance of giving it a scrub before the experiment. That is how it is discharging. Could this experiment still work if the zinc plate was surrounded in a gas that prevented oxidisation?
Ответить
I think it is bit of misleading, the rod was originally negatively charged....otherwise you won't get negatively charged plate by induction
Ответить
Thank you for the demonstration sir.
Sir, I have a question. How rejected electrons be replaced under photoelectric effect.?

A good teacher you are
Ответить
this video sux!!!
Ответить
Just by looking at electron deflection behavior, arriving at particle notion seems very crude. Increasing the Intensity of the wave may not deflect electrons out of the metal, because electrons also possess wave behaviour. Electrons are particles with wave characteristics at the outskirts of their self spin. Although visible light may not deflect electrons out of the metal, it still energizes them.
Ответить
It finally clicked ! Thank you this helped me understand this important concept
Albert Einstein was the fucking man dude

this is crazyyyyyyyyyyyyyyyyyyyyyyyyyy...............................................
Ответить
thank you so much for this! i got a test tomorrow and the fact that this video gets straight to the point really helps. <3
Ответить
This is beyond explicit! Thank you so much!
Ответить
----I believe it is called the photo-electric effect , when a photon acts on a reacting electron , knocking it out of orbit , correct ?
How can an electron be forced out of orbit after being struck by a force without mass ?---

How can we be sure that it's not discharging because the big metal UV light is near it? Similar to the rod but opposite charge?
Ответить
This is a great video !
Ответить
Let me put this effect in simplest terms. Electrons can only be removed from a nucleus and emitted by absorbing discrete packets of energy. Electrons cannot absorb continuous amounts of energy. If they could, any wavelength of light could remove the electrons. Instead, only some wavelengths can remove electrons and not all. This must mean that, one, electrons cannot absorb continuous energy, two, light is made of discrete packets of energy, and three, electrons can only absorb discrete packets of energy. Also, if you’re confused that I said electrons can only absorb particles and yet electrons can only absorb certain wavelengths, all I mean is that if you perform the double slit experiment, and record each wavelength measured for all types of electromagnetic radiation emitted, and then consider the different types of light shined in the photoelectric effect to be the wavelengths simply to differentiate what sort of light is being shined in the experiment, it is useful.
Ответить
Can someone please explain how did the plate get negatively charged in the first place?
Ответить
Great video 😍😍
Ответить
Is it true that american army has photoelectron weapon that has an effect on brain?( x-ray laser capable of conveying electron )
Ответить
Muito bom , me lembrou minhas aulas na faculdade de Física, todo experimento é válido e de alguma forma os de Física são muito belos e harmoniosos parabéns
Ответить
just eaten a bagel
Ответить
Does anyone confuse about the induction part? I suppose the glass rod hold in the hand is negatively charged after rubbing with the cloth (not positively charged mentioned in the video), so the metal plate can be positively charged after induction. Then the electrons will flow to the metal plate when it is earthed. The whole process can end up with exceed of electrons remaining on the metal plate and leaf.
Ответить
60 dislikes are ppl who are failing their classes
Ответить