Комментарии:

Best minute and a half of my life! For being such a simple compound, I was baffled for an embarrassingly long time trying to figure out what to do with it
Ответить
❤
Ответить
I thought there was a coordinate bond too
Ответить
I really appreciate your brilliant explanation. Although my mother tongue is not English but I understood because you explained perfectly. Thank you so much
Ответить
10 years of helping a kid
Ответить
Beautiful explanation
Ответить
Wrong
Ответить
Wow, thank you for the video Sir.
Ответить
I can't understand!! Please help me..
Ответить
I was confused about this alot
Thank you so much

your explanation was shite
Ответить
10 Years later, this video is still helping students. Thank you.
Ответить
It is wrong structure
Ответить
Thank you sir my school teacher couldn't explain it properly
Ответить
Thanks ❤
Ответить
That's the fukkin' way she goes, boys.
Ответить
Does no one realize how this is completely wrong
Ответить
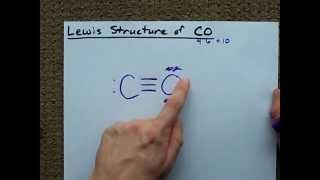
I honestly still don't understand because one bond represents two electrons bonded , one from oxygen the other from carbon....in this case we have 3 bonds meaning 3e from oxygen bonded with 3e from carbon and when you add the electrons around the C atom it now sums up to 5e when include the lone pair of electrons or are we suppose to assume that the electron pair from the O atom was shared amongst the two atoms and the two atoms didn't meet each other half way such that one atom brings one and the other brings one to form a bond?
Ответить
Best way of explaining!
Ответить
But o to 2bond karta h
Ответить
Thanks 👍
Ответить
What is about charge produced on the atoms in molecule?
Ответить
shouldn't CO have 2 coordinate bonds then ?
Ответить
its wrong totally wrong
Ответить
Good explanation thank you
Ответить
This seems wrong as the structure that you have drawn shows five electrons in carbon’s valence shell and again five electrons in oxygen’s valence shell, which is not possible right ?
Ответить
Lots of love from India🇮🇳🇮🇳🇮🇳🇮🇳🇮🇳
Ответить
Nice
Ответить
Thank you sir
I don't know English very well
But I understand this very easily ❣️

this bonds is so wrong in the tet books , cause this is not a covalent bond , there are coordinate bonds as well here , there is a covalent double bond and 2 coordinate bonds ,
Ответить
the first bond is a coordinate bond as oygen dosent share anything
Ответить
Thanks a lot.
Ответить
You a badass man
Ответить
thanks buddy
Ответить
Great
Ответить
Legend are watching 2022
Ответить
But wait :q Now oxygen and carbon both still have formal charges :q Shouldn't we cancel them? :q
Ответить
Oh I couldn't understood this before thnaku very much for ur clear explain ♥️
Ответить
You save my life! Thank you!!!!! You explained very very well
Ответить
Thank you very much
Ответить
explained ina minute what my chem. professor didn't manage to do in 2 hours
Ответить
This got me through some tough times thanks chemist Nate I luv you
Ответить
What about formal charge? Doesn’t the carbon need only 4 electrons right around and the oxygen needs only 6 electrons right around it?
Ответить
why dont u make 4 pounds betwean them and o (oxcegen) take the left pair ????....please tell me I need help
Ответить
Hi
Ответить
But how do you know to automatically fill oxygen with as much as possible first? Because it's more electro negative? Is this always the case? What I mean is, why not start out with the single bond, then putting only 5 around oxygen and 3 around carbon as would be according to their respective valence electrons?
Ответить
Thank you 🙏🙏🙏
Ответить
But how oxygen can make 3 bonds?
Ответить
Thanks
Ответить
Wow❤️
Ответить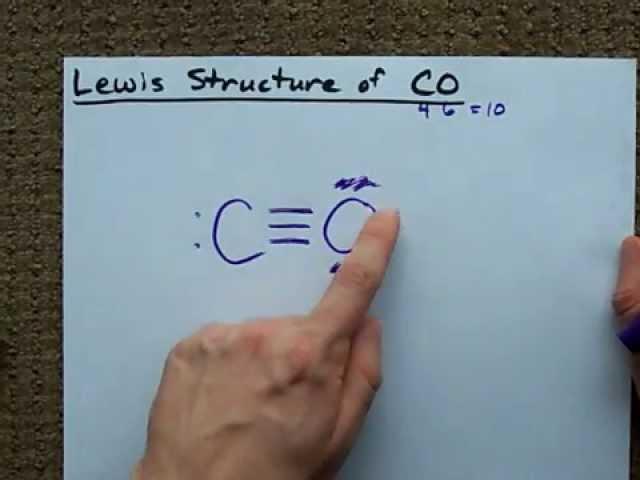
![Trippie Redd - Supercell [Instrumental Remake] (BEST VERSION) Trippie Redd - Supercell [Instrumental Remake] (BEST VERSION)](https://invideo.cc/img/upload/RW9IT2NHWkt0WjY.jpg)