Комментарии:

“Kindaaaaa saaad” -Matt Damon
Ответить
Thank you. My teacher is terrible at explaining
Ответить
boring no offense but you just make this all boring
Ответить
Super helpful! Thank you! I drew my first Lewis dots on my own from watching your videos!
Ответить
Your such a good teacher. 👍🏽👍🏽👍🏽 wish my teacher was like you 🙄🙄🙄 thank you 🙏🏽
Ответить
Thank you so much! This was totally better explained and easier to understand than what I had been taught (and trying to understand). Thanks!!!
Ответить
All those other vids I wasted my time on watching and here is the answer.
Ответить
Lewis needs the Nobel prize.
Ответить
So we only complete the octet rule for the atom in the middle???
Ответить
My chem teacher teaches this differently. You wouldn’t start at the top, but on the right side.
Ответить
Why do teachers gotta make this kind of stuff complicated?
Ответить
Thank you
Ответить
Why aren't you verified?
Ответить
anyone else just seeing this now
Ответить
Why is He in the 8 column tho it only has 2 electrons at the outside(´ ・ω・`)?
Ответить
God bless you
Ответить
RIP Dr. Lewis
Ответить
wow..thank u very much...
Ответить
can you pls teach my teacher aswell
Ответить
intelligent ppl really are attractive <3
Sensei, daisuki ♡

10 years later and this guy is still helping students through these videos, how amazing! thanks dude <3
Ответить
mr.Anderson you are a life saver
Ответить
The wry laura functionally hammer because attempt decisively retire save a hard salad. harmonious, glamorous wasp
Ответить
Now try that with nitrogen dioxide ;>
Ответить
When writing the chemical formula for two elements together, does the element with the bigger charge go first?
Ответить
Hunds rule
Ответить
Why you are putting dots 1 by 1 instead putting 1 pair of dot first then for the third dot just starts with 1 dot
Ответить
Can nobel prizes be awarded post-humously?
Ответить
Your such a giga chad
Ответить
Thanks Mr. andersen you help me pass my physiology class now im in chemistry still watching your videos
Ответить
Damn it’s 2021, this video still helping students….
It show that he’s a good teacher (I presume)

Thanks homie
Ответить
u were the reason I passed AP bio with an A sir. now im in college studying for my chem final and remembering my roots
Ответить
Nice teaching trick😀
Ответить
Dude you've been a staple of my classes since freshman year of high school and you're still helping me with college classes. You are the MVP of my science education.
Ответить
Thank you
Ответить
thank you
Ответить
Thanks man
Ответить
Good
Ответить
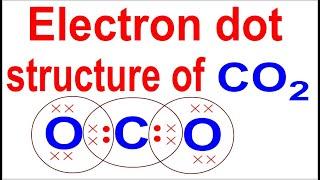
Anyone else noticed the SiLiCON at the bottom?
Ответить
i find it funny he spelled out silicon with Si, Li, C, O, N
Ответить
Tq so much 😊 sir for explaining about Lewis dot structure ❤
Ответить
tysm you saved me
Ответить
I wonder why this technique wasn't patterned to configure the s pair first, next adding one dot each orbital for p (one spin), and then pairing the p orbitals after 1 dot is in each (opposite spin)? Maybe that wouldn't work well for depicting molecules and compounds?
Ответить
First few seconds says C.S Lewis. Literally encouraged me to keep studying
Ответить
this is great, thank you
Ответить
It's 2024, almost 2025 and you're still the best!
Ответить







![What Are The Odds This Happened On My Birthday?? | Free 2.0 Succeed [332] What Are The Odds This Happened On My Birthday?? | Free 2.0 Succeed [332]](https://invideo.cc/img/upload/VWU4RlBMZ2lXVUk.jpg)