Комментарии:

U can't imagine how greatful I am thank u sir
Ответить
But isn’t lactose~ beta-d-galactose + beta-d-glucose?
Ответить
amazing..... didn't need to read the Uni content at all after watching this !
Ответить
Thank you very much!!
Ответить
You explain very well, 🙂🙂
Ответить
thank you so much,, for explaining.. I had a great experience Watching 😊
Ответить
Wouldn't for sucrose, you would need to specific alpha-1,-beta-2-glycosidic bond, since it's using both anomeric carbons?
Ответить
I'm writing a comment here for my own reference in the future. No other places to take notes right now. '
Aldehydes can be oxidized into carboxylic acids, which are also formally called aldonic acid.
Because maltose and lactose have 1,4-glycosidic bonds (regardless of their alpha or beta conformations), this means that there is still at least one anomeric carbon capable of opening up into the open-chain aldehyde form (also known as mutarotation). Thus, it is called a reducing agent (reducing sugar).
And sucrose is not a reducing sugar because the anomeric carbons are both taken up in the alpha-1,2-glycosidic linkage, meaning that there's no chance for aldose formation....? Isn't beta-D-fructose a ketose though?
I did a bit of googling and I found that ketose sugars can tautomerize (rearranging bonds) resulting in an (enol), a double bond and -OH group. So ketoses also act as reducing sugars by becoming aldoses until a "keto-enol" shift under basic conditions...interesting.
aldoses = open chain carbohydrates with aldehyde as their oxidized functional group
ketoses = open chain carbon hydrates with ketone
hemiacetal = cyclic rings due to intramolecular reactions between an nucleophilic hydoxl group and electrophilic carbonyl carbon in an aldose

Your lectures are so much informative and fantastic even for teachers
Ответить
Lactose=alpha galactose +beta glucose ???
Ответить
Thank you!!! Finally, I understand this.
Ответить
I have understood so much thank you
Ответить
Thank you!!! Finally I understand this.
Ответить
What is the full name of lactose?،🧐🤔
Ответить
U are genius 😎
Ответить
In fructose, hydroxyl group in C3 is in the opposite direction as others, as a result, OH resides inside the pentagon ring in C3. But when it becomes Sucrose, i noticed that hydroxyl group is inside of pentagon in C4. Can you please explain why?
Ответить
Your explanation is simply tremendous 👍
Ответить
Thanks alot
Ответить
I found this verrry helpfull as a med student thanks
Ответить
I'm in love w your handwriting 😍😍❤️
You are so organised

these videos are so clear and helpful, thankyou so much
Ответить
Thanks sir
Ответить
Thanks sir ☺️
From india

thank you Andy
Ответить
Thank u... Love from Bangladesh
Ответить
Literally my savior for over 3 years now. THANK YOU
Ответить
Hello sir. Is it supposedly a-D-fructose + a-D-glucose to create a-1,2-glucosidic bond?
Ответить
Great lecture! Made me really understand the structure rather than basically memorizing them without any context
Ответить
That board scares the hell out of me and get me lost why not try with less written stuff up on there
Ответить
Thhhhankkkk you very so alot much 😁
Ответить
Well explained ☺
Ответить
very well put together lecture.
Ответить
just got it now... thank you
Ответить
Really helpful! Thank you!
Ответить
Sell your tshirts so we can support you !
Ответить
we want more video's like this
Ответить
6. If a disaccharide is formed through a (1-> β4) glycosidic bond, it means that ______.
A. neither the first nor the second monosaccharide unit needs to be in the β-form
B. both monosaccharide units need to be in the β-form
C. only the first monosaccharide unit needs to be in the β-form
D. None of the above is correct.
Can someone help me with this qn?? )):

thank you so much !
Ответить
good
Ответить
The reason the maltose C1 anomeric carbon is designated alpha is that it's former hydroxyl group, which is now the glycosidic O bond linking the two monsaccharides, is cis to (on the same side of the ring) as the 4th carbon's hydoxyl group. The lecturer was wrong when he referenced it to the position of the CH2OH group attached to carbon no. 5. That group is trans to the glycosidic O bond. The alpha/beta designations refer to the positions of the anomeric hydroxyl group and the hydroxyl group oxygen which is directly bound to the highest numbered chiral carbon in the ring.
Ответить
α-glucose + α-fructose = sucrose
Ответить
and maltose has alpha and beta glucose
monosacharides.

Lactose has both galactose and glucose in beta anomeric conformation not in alpha glucose
Ответить
Well a great video lecture.....it really built up my concepts
Ответить
thank you!
Ответить
Also greetings from Germany. I'm taking Biochemistry in German (not my first language) and your videos really are a life-saver for the concepts that are harder for me to translate. Much appreciated!
Ответить
greetings from Germany I understood so much more in your Video than I understood in German videos or in class thank you so much🙌
Ответить
Your strong pronunciation is great really learning here
Ответить
isn't lactose between beta galactose and BETA glucose?
Ответить
Thank you very much. I search for hours for a nice german statement. But its so much easy, when u explain it.
Ответить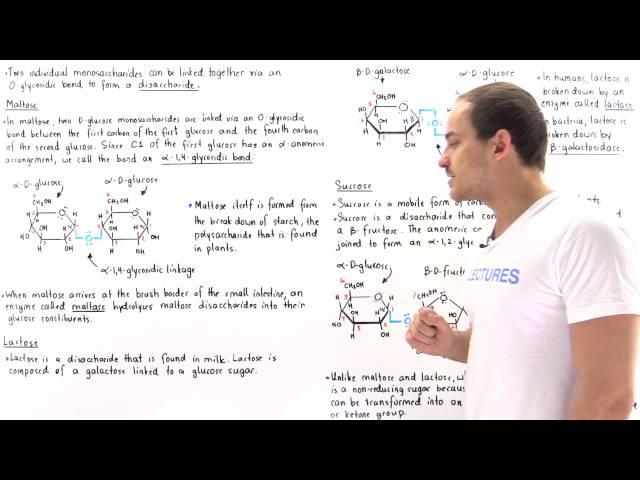




![[LIVE] Video tutorial edit with Blender 3d and Power Sequencer [LIVE] Video tutorial edit with Blender 3d and Power Sequencer](https://invideo.cc/img/upload/RTRGUkROYnRMVnU.jpg)